A Hitchhiker's Guide to ZULF NMR
A broad overview
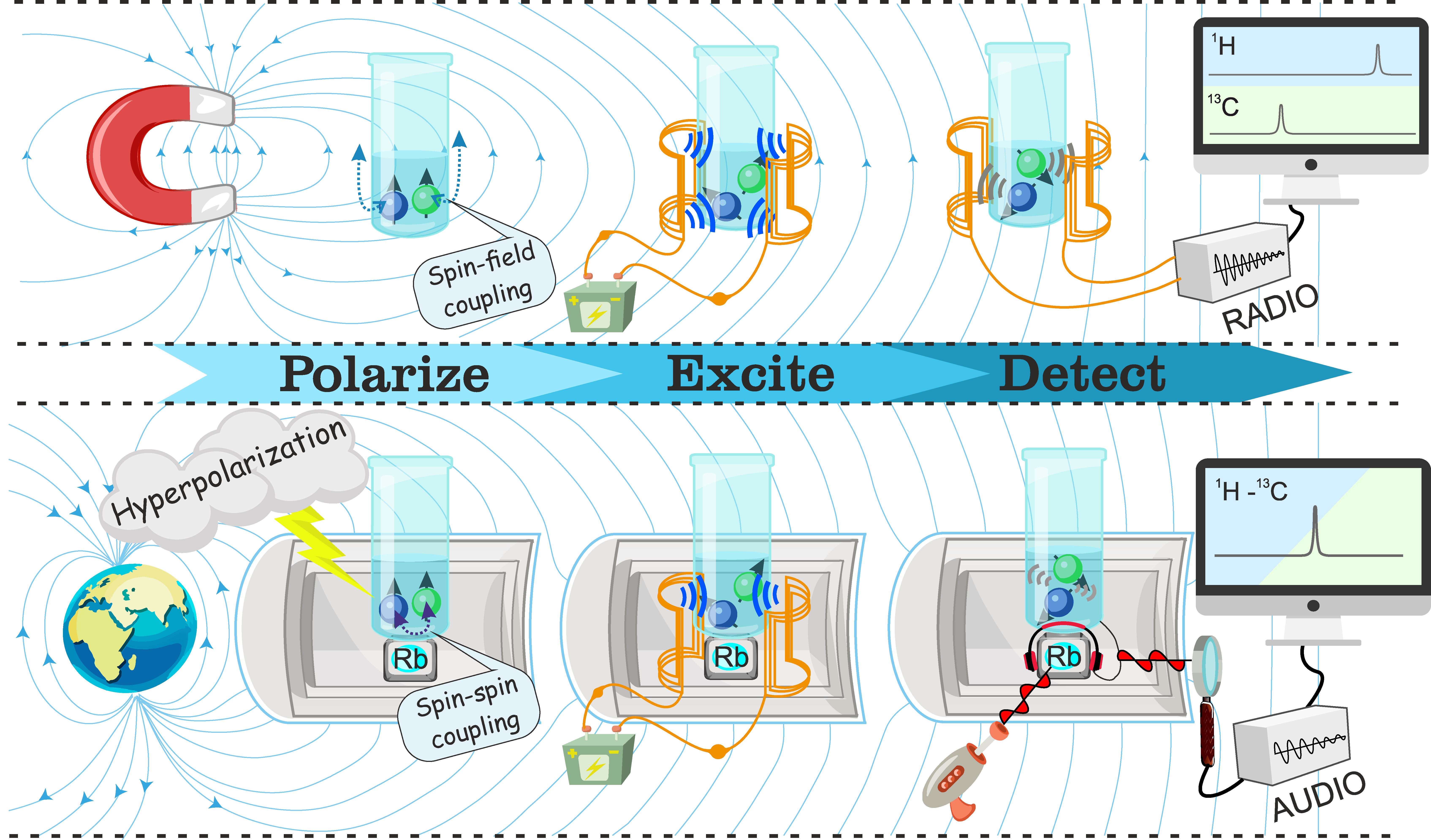
Nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) are research fields which, broadly speaking, concern the interaction of nuclear spins with magnetic fields. This can be a large external magnetic field, smaller magnetic fields generated within molecules, or the magnetic field produced by a second nearby nuclear spin, to name a few examples. These interactions between nuclear spins and magnetic fields allow an enormous range of applications, from chemical recognition to structural imaging of the human body, from the elucidation of protein structure and internal dynamics to chemical reaction monitoring, and from metabolic imaging in living organisms to the study of fundamental physics. In many areas of magnetic resonance research the prevailing wisdom is 'the larger the field the better', and for good reason: this generally increases the amplitudes of the NMR signals, and enhances chemical shift resolution in spectra. Most modern NMR magnets operate with a field of ~1-30 T, but this comes at a cost. For any field beyond a few tesla, expensive cryogenics are usually required to cool down the superconducting coils, and this renders the magnet bulky and far from portable or compact.
An alternative is to go in the other direction and reduce the size of the magnetic field with magnetic shielding, all the way to zero. At zero-field, there is no longer an external field for the nuclear spins to interact with, which means we have two significant drawbacks: (1) we lose chemical shift information in the spectra, and; (2) we must provide an external source of nuclear spin polarization, because in contrast to high-field NMR there is no large field to polarize the spins. The loss of chemical shift information is tolerable, since the spin-spin interactions (J-couplings, dipolar couplings, quadrupolar couplings..) remain, and are a rich source of information on the structure, dynamics and distribution of molecules in space. The need for an external source of sample polarization is met by either pre-polarizing the sample in an external magnetic field (upcoming Blog Post 2), or by employing one of a large number of established and emerging hyperpolarization techniques (upcoming Blog Posts 3, 4, 5, 6 and 7 for more information). The door is then open to performing NMR experiments at zero- and ultralow- magnetic fields! But why?
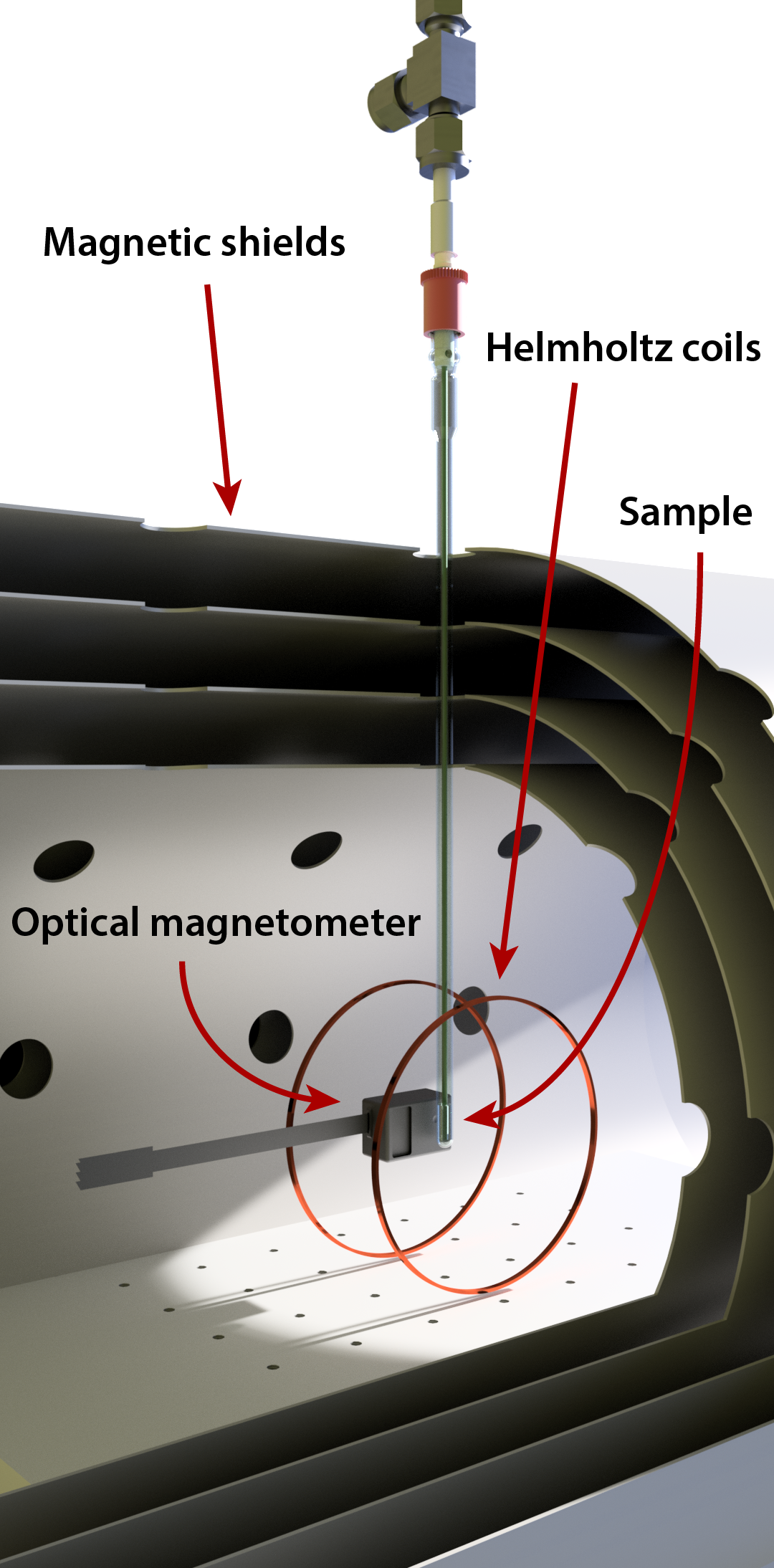
Figure 1: A schematic of a ZULF experiment.
This is an exciting new regime to operate in for a number of reasons.
- The spectrometer which uses a few layers of magnetic shielding can be much smaller, and hence more portable, than the high-field analogue (upcoming Blog Post 8)
- There is no magnetic susceptibility- induced line broadening in the spectra of inhomogeneous or biphasic samples (upcoming Blog Post 9)
- The skin depth of electrically-conductive materials (i.e. most metals and alloys) is much higher for the low-frequency signals acquired in a zero- to ultralow-field (ZULF) experiment, meaning the experimenter can probe through metal, which is generally not possible at high-field
- Certain physical interactions are suppressed in high-field spectra, but become visible at zero-field (upcoming Blog Post 10)
The experimental setup is relatively simple, and a schematic is shown in Figure 1. A zero- or ultralow-field region can be created inside layers of mu-metal shielding, which attenuate the ambient magnetic field by a few orders of magnitude. The residual field can be further reduced by ‘shimming’, which is to apply compensating magnetic fields via coils on the inside of the mu-metal chamber (note that these shim coils are not shown in Figure 1). A signal can be excited in the sample by applying brief magnetic field pulses; a pair of Helmholtz coils is shown for this purpose in the schematic. The signal is then measured, typically with an atomic magnetometer (a QuSpin magnetometer is shown in Figure 1), which is a highly sensitive device to detect the magnetic fields produced by the nuclear spins in the sample. A more detailed description of the ZULF experiment will be given in blog post 2.
This technique has two advantages. For a start, we are able to analyze samples in metal containers and, at the same time, we can examine more complex substances made up of different types of components. We think our concept could be extremely useful when it comes to practical applications.
Professor Dmitry Budker
What exactly is meant by zero- to ultralow- field?
In this blog series, and in literature on the topic, we typically talk about the regimes zero-field, ultralow-field, low-field and high-field. We define these terms for solution-state experiments using nuclear spin parameters specific to the system being studied: J-couplings (JII and JIS), homonuclear chemical shift differences (δI1 - δI2), heteronuclear gyromagnetic ratio differences (γI - γS), and characteristic spin relaxation times (Trelax). Figure 2 displays the different field regimes, our definitions for the boundaries, and their physical significance.
Figure 2: A schematic showing our definitions for the different field regimes. The boundaries calculated clearly vary significantly depending on the system under study.
Note that for a sample without measurable J-couplings (e.g. a bulk sample of water), the only regimes are high- and zero-field; it would be meaningless to talk about low- or ultra-low fields in this case. It is also important to note that in other areas of research different naming conventions and definitions are used, and our definitions would also need modification if dipolar or quadrupolar couplings were considered. Even with these broadly defined regimes there are special cases; for example, blog post 11 will discuss Earth-field NMR, which is a type of NMR experiment detected in the ambient lab field.
So what's the point in all of this?
ZULF NMR allows us to access a new range of applications that regular high-field NMR is ill-suited to tackle. Spin-spin interactions can be explored in fundamentally different ways, the apparatus is cheaper and easier to transport and adapt to specific applications, and it is more feasible to study samples that are close to, or enclosed by, metal objects. In addition, without a large magnetic field present, there is no loss in spectral resolution associated with inhomogeneous samples and complex media.
Many important ideas have been brushed over in this post, and the upcoming posts in this blog will meander through these topics in greater detail. This blog will hopefully provide you, the reader, with a flavor for this emerging spectroscopic technique, as well as some insight into related topics.
Title image creators: Dr. Laurynas Dagys, Ms. Seyma Alcicek

